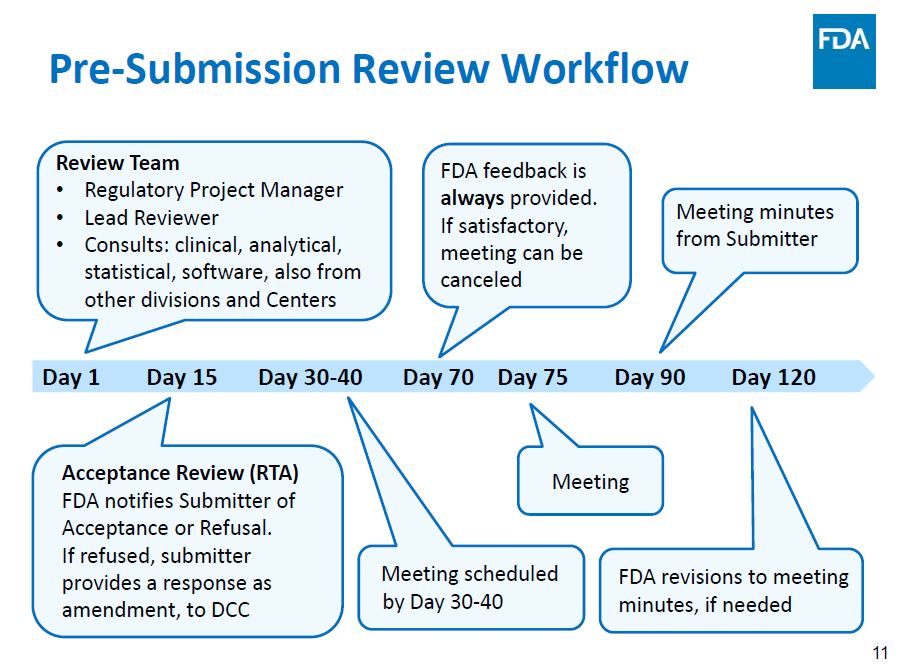
OxiWear submitted its FDA 510K (II) pre-submission package on November 12 with the assistance of MethodSense, its FDA filing consultants. The company received receipt confirmation along with its official FDA docket number. The team is scheduled for its pre-review meeting in early February 2022.
If you are interested in reading about the full process, you can find more information at FDA: The Pre-Submission How to Efficiently Communicate with FDA About Planned Applications.